
Estructura de Lewis NH3, Amoniaco » Quimica Online
Ammonia NH3 Lewis Dot Structure shadowboy220 1.9K subscribers Subscribe Subscribed 49 Share 16K views 11 years ago Chemistry Lewis Dot Structures A video explanation of how to draw the.
Ammonia Molecule Photograph by Molekuul/science Photo Library Pixels
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Ammonia, anhydrous ammonia, uses, levels, test & ammonia health effects
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale.
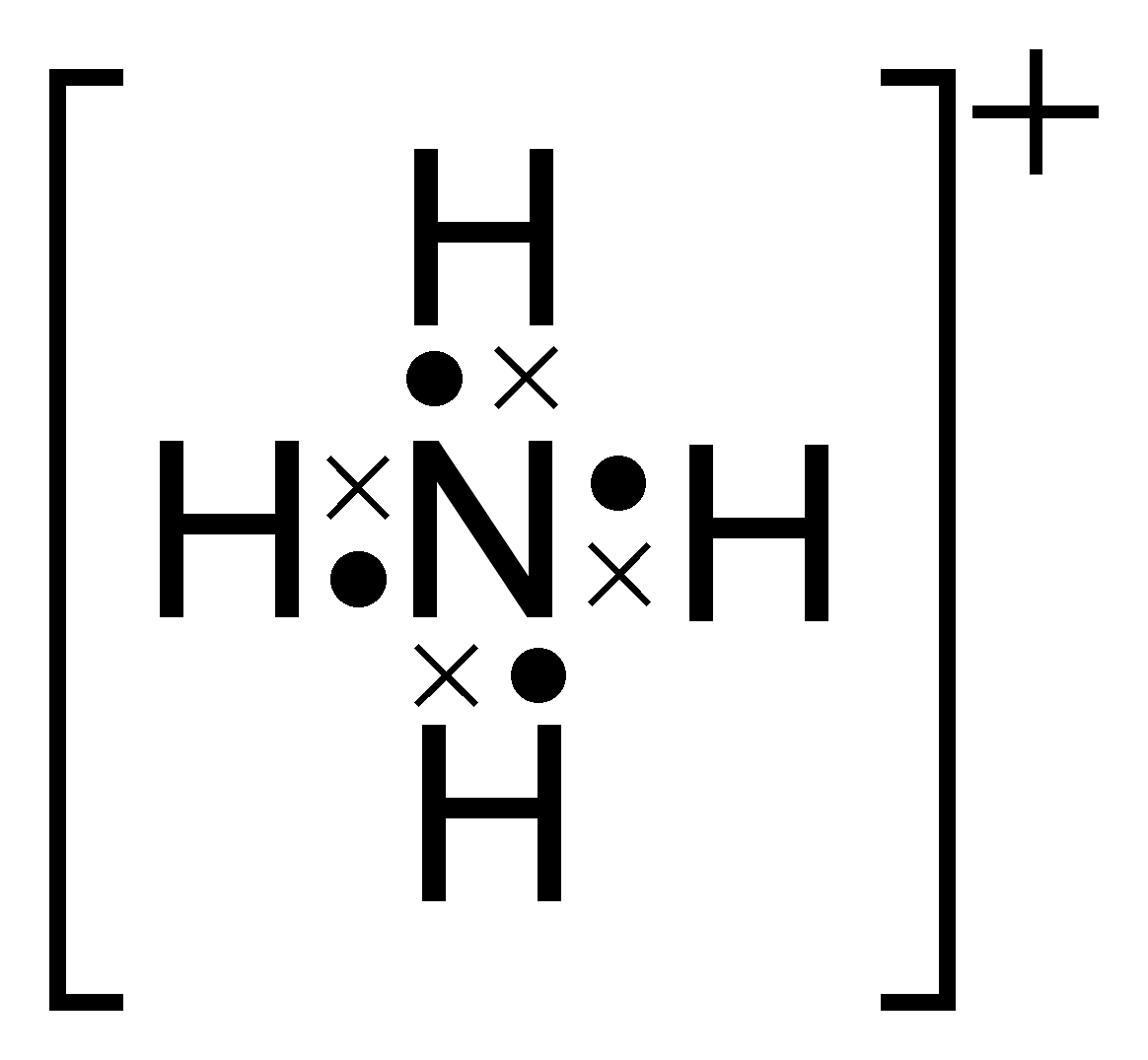
Electron Dot Diagram Of Ammonium Ion
It consists of hydrogen and nitrogen. In its aqueous form, it is called ammonium hydroxide. This inorganic compound has a pungent smell. In its concentrated form, it is dangerous and caustic. The NH 3 chemical name is ammonia. Table of Contents Ammonia Structure Preparation of Ammonia Properties of Ammonia Uses of Ammonia

Estructura de Lewis NH3, Amoniaco » Quimica Online
Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.

Ammonia Formula Structure, Formula, Properties, Preparation Embibe
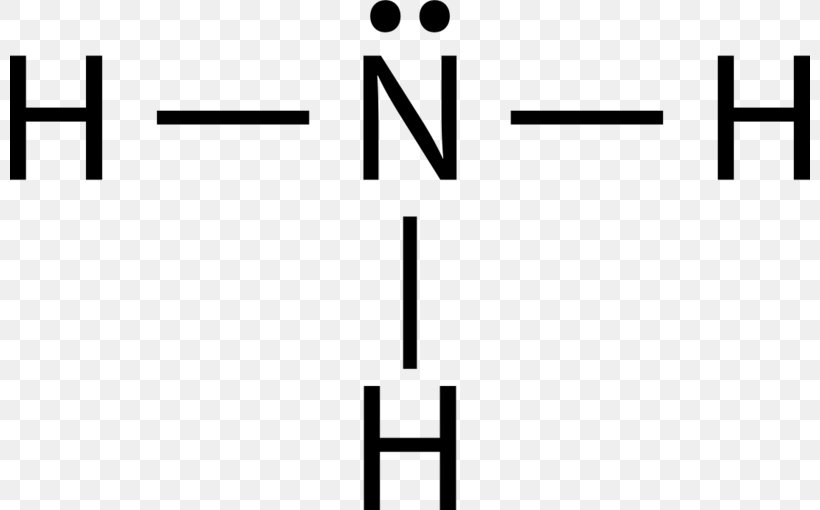
In the Lewis structure of ammonia, the nitrogen atom forms three single bonds with the hydrogen atoms and has one unshared electron pair. Ammonia Formula. The chemical formula for ammonia is NH3. The formula indicates that there are three hydrogen atoms and one nitrogen atom in each molecule of ammonia. The formula represents the composition of.

Ammonia Formula NH3 Equation, Formation, Structure, Charge
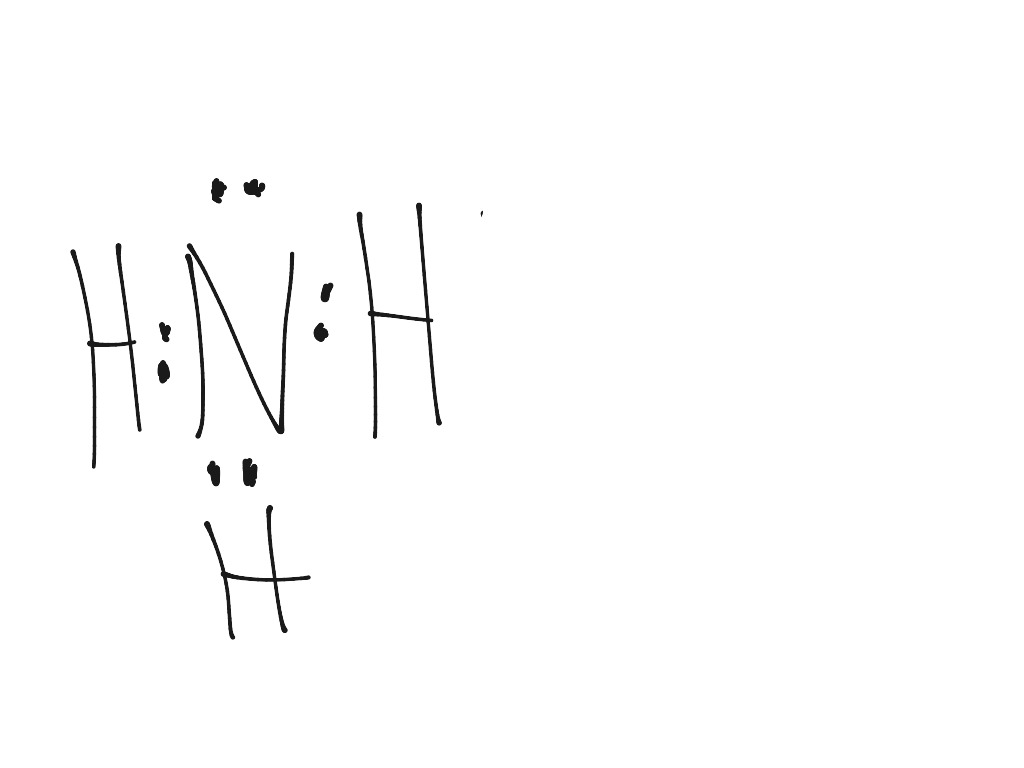
Lewis structure of NH3 (ammonia) contains three single bonds between the Nitrogen (N) atom and each Hydrogen (H) atom. The Nitrogen atom (N) is at the center and it is surrounded by 3 Hydrogen atoms (H). The Nitrogen atom has one lone pair. Let's draw and understand this lewis dot structure step by step.
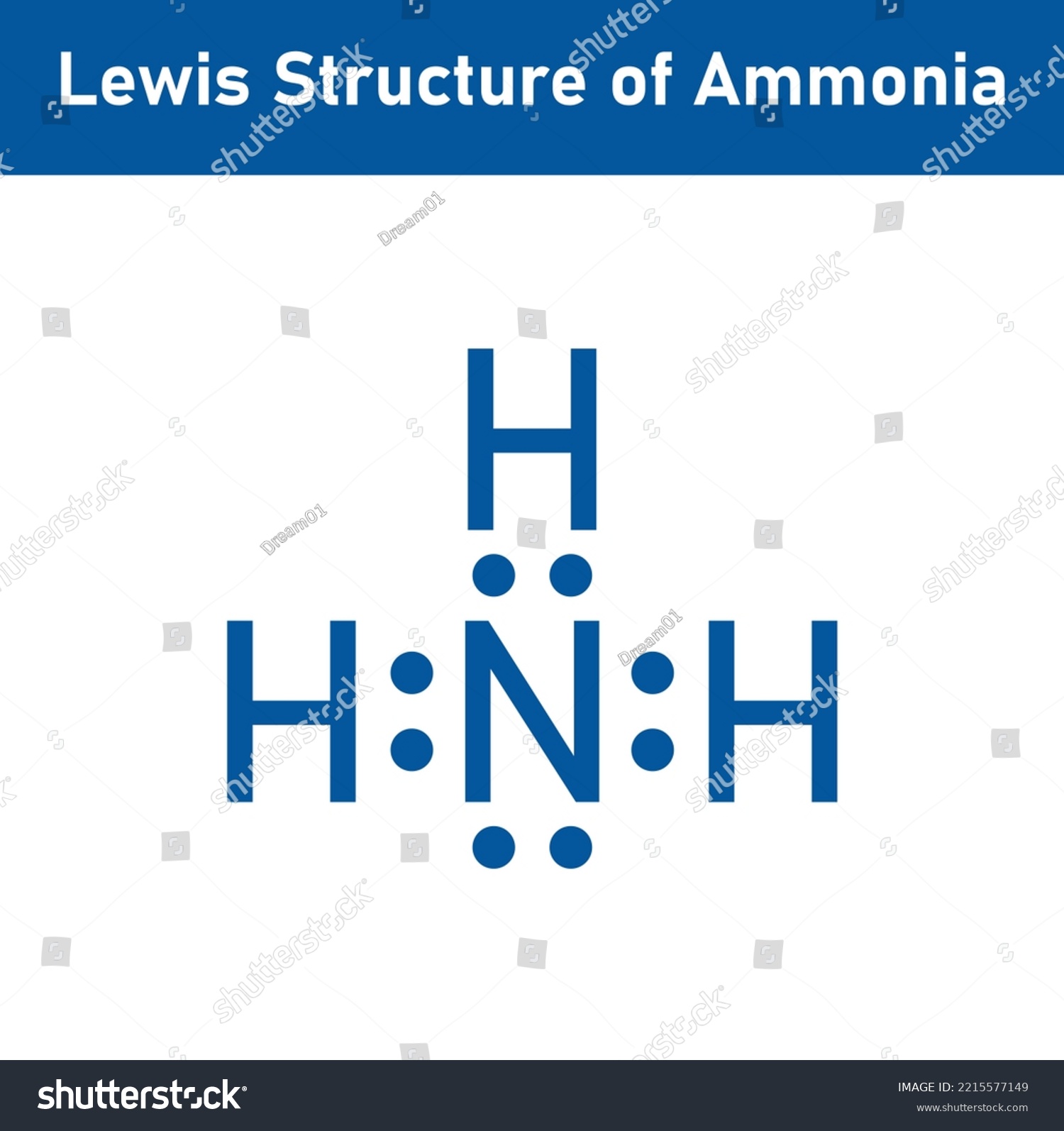
Lewis Structure Ammonia Nh3 Scientific Vector Stock Vector (Royalty Free) 2215577149 Shutterstock
Learn the steps to draw the Lewis Structure of NH3 (ammonia) in just 1 minute.📌You can draw any lewis structures by following the simple steps mentioned in.
Ammonia Lewis structure Science, Chemistry, Chemical Bonds ShowMe
In the lewis structure of ammonia (NH 3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial.

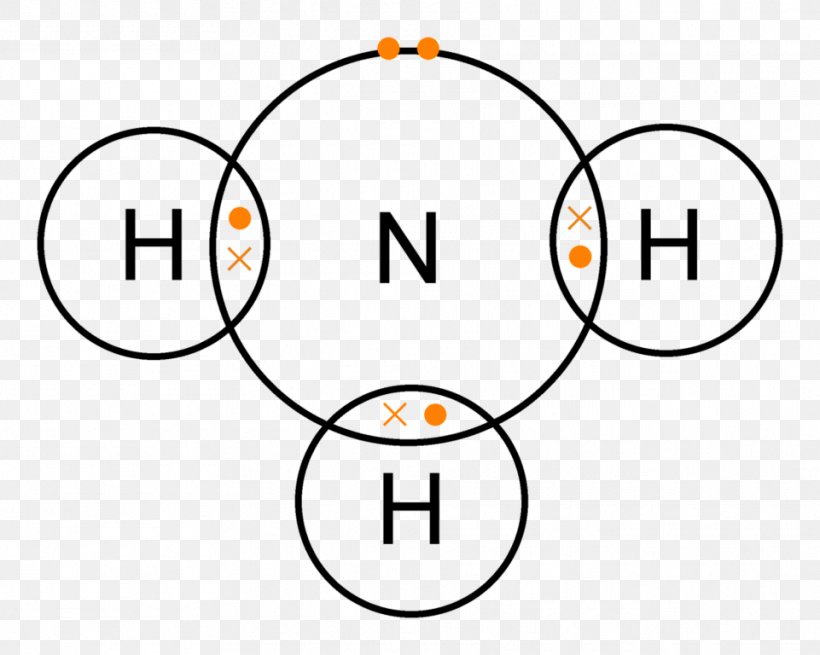
FileAmmonia lone electron pair 2.svg Wikimedia Commons
Steps involved in the NH3 Lewis structure: Step 1: Valance Electron Determination: Step 2: Central Metal Atom: Step 3: Connect the Atoms with a lone pair: Step 4: Distribute Lone Pairs: Step 5: Complete the Lewis Structure: Step 6: Formal Charge: Detailed applications: Synthesis/Production:
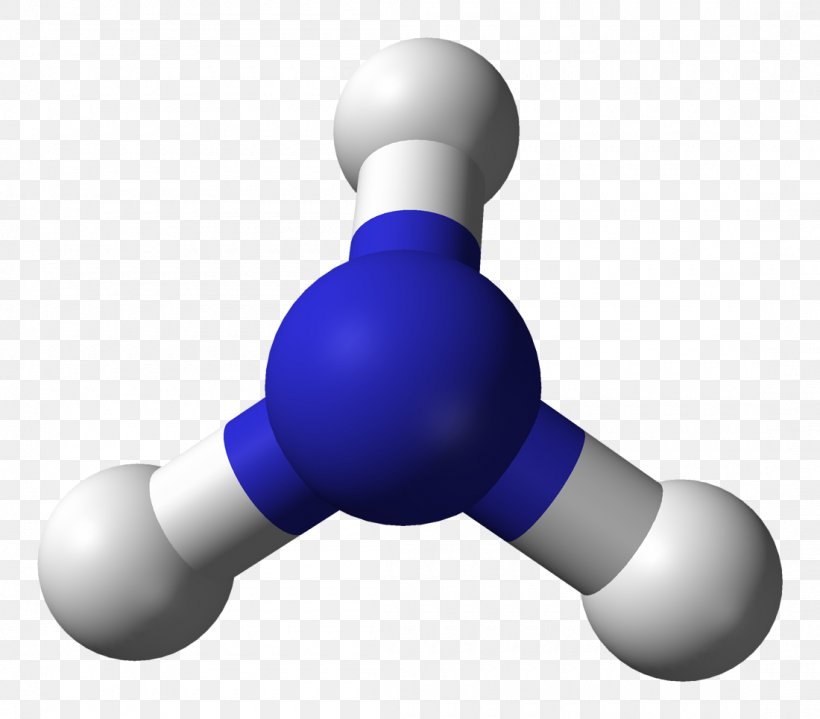
Ammonia Molecule Molecular Geometry Ballandstick Model Lewis Structure, PNG, 1100x965px
Ammonia (NH3) lewis structure is made up of one nitrogen (N) atom and three hydrogens (H) atoms. In, lewis's structure of NH3, three bond pairs, and one lone pair are present. The nitrogen (N) atom is situated at a central position and the hydrogen (H) atoms are at the outside position in the lewis diagram.
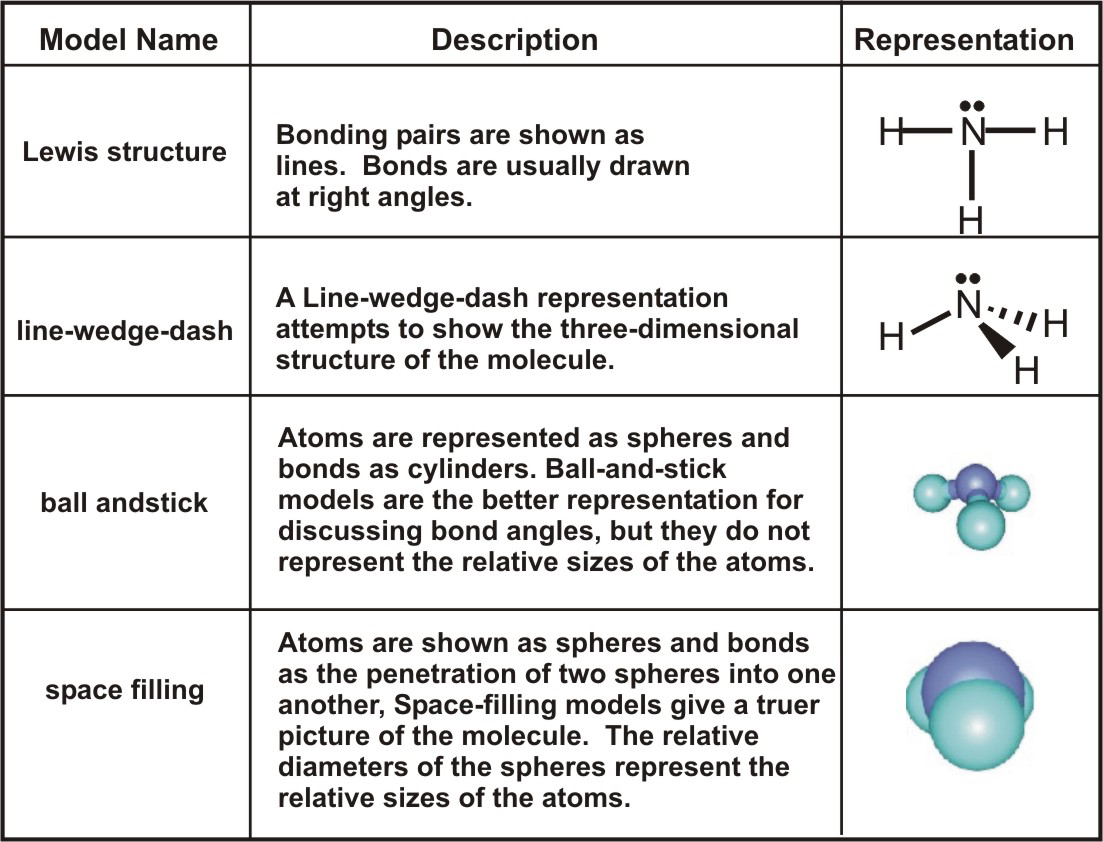
Chapter 6 Molecular Structure
1: Structure and Bonding 1.3: Lewis Structures Expand/collapse global location 1.3: Lewis Structures Page ID Using Lewis Dot Symbols to Describe Covalent Bonding
Lewis Structure Ammonia Lone Pair Ammonium Lewis Pair, PNG, 800x510px, Watercolor, Cartoon
Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. Video: Drawing the Lewis Structure for NH3 It is helpful if you:
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG, 961x768px, Lewis Structure
Ammonia (NH 3) Molecule Shape, Geometry, Hybridization. Ammonia lewis structure contains three sigma bonds and one lone pair on nitrogen atom. Therefore, there are total of four electrons regions. So, hybridization of center atom, nitrogen is sp 3.Because there are four electrons regions, geometry is tetrahedral and shape is trigonal pyramidal.

The Shape of the Ammonia Molecule Nh3 Is
Understanding the NH3 Lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. By following the step-by-step guide provided in this article, you can draw the NH3 Lewis structure accurately and gain insights into its molecular bonding.
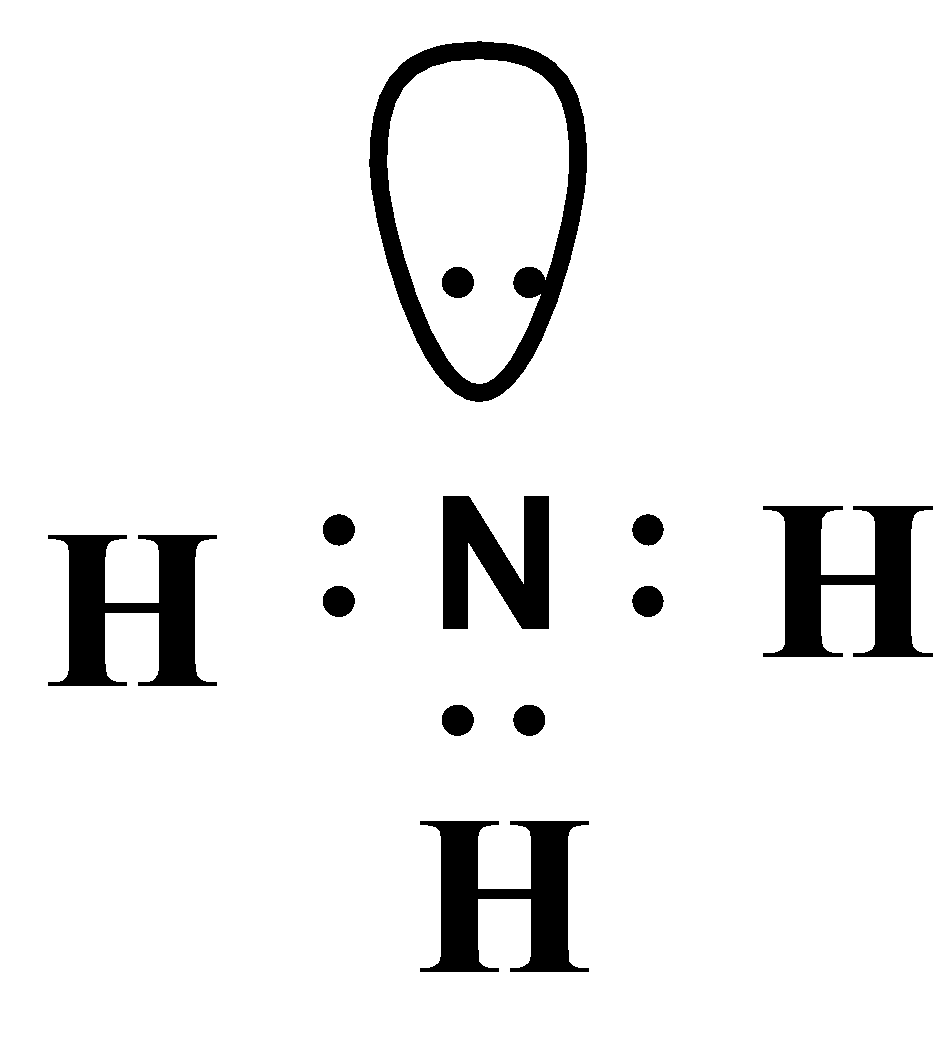
How is ammonia represented by an electron dot diagram?
This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia.Chemistry - Basic Introduction: https:.